Software as a Medical Device to help personalize care | Takeda Stories

3 ways we’re using Software as a Medical Device to personalize care
Syringes, inhalers, transdermal patches, nasal sprays – as a pharmaceutical company, we often develop physical medical devices to complement our therapies. And now, with the rapid advancement of digital capabilities, certain digital solutions can support diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease. These Software as a Medical Device (SaMD) solutions are regulated to ensure they are reliable and accurate for patient safety.

Hélène Haarmann, Patient Health Excellence lead
SaMD solutions are rooted in a deep understanding of unmet needs. Our Patient Health Excellence team analyzes the patient journey and the health care ecosystem in specific therapeutic focus areas and identifies pain points that, if addressed, could improve patient outcomes. “We work cross-functionally with Takeda stakeholders and collaborate with patients and health care professionals to identify and prioritize potential solutions to improve the patient experience,” Hélène says.

“There is a growing need for Software as a Medical Device to complement our medicines, either for therapeutic purposes or for supplementing monitoring and diagnostics. This is where the future lies.”
Sriman Banerjee, executive director and head, Diagnostics, Software Devices & Packaging Development, Takeda
It’s an approach that has begun to reap rewards over the past several years. As the examples here illustrate, our SaMD innovation has led to software solutions to help patients and clinicians in conditions such as Crohn's disease and hemophilia, where patient management can be challenging.
Sriman Banerjee, executive director and head, Diagnostics, Software Devices & Packaging Development, says: “There’s a growing need for SaMD to complement our medicines, either for therapeutic purposes or to supplement monitoring and diagnostics. This is where the future lies. And the potential grows as the technology underpinning it grows.”

Tim Moloy, Connected & Software Devices lead
Tim Moloy, Connected & Software Devices lead, adds: “It’s exciting to see the many algorithms and tools that touch a patient’s life, operating through smartphones in their pockets. The blend of medical devices with consumer electronics is a natural progression, and we’re using these advancements to innovate for patients and providers as we’ve done throughout our 243-year history.”
Here are three ways we’re using SaMD to personalize patient care:
Helping predict likelihood of treatment response in Crohn’s disease
There are a growing number of treatment options for Crohn’s disease (CD), which can make selecting the right treatment for a patient challenging. Additionally, fewer than one-third of CD patients treated with advanced therapies respond to their treatment.1
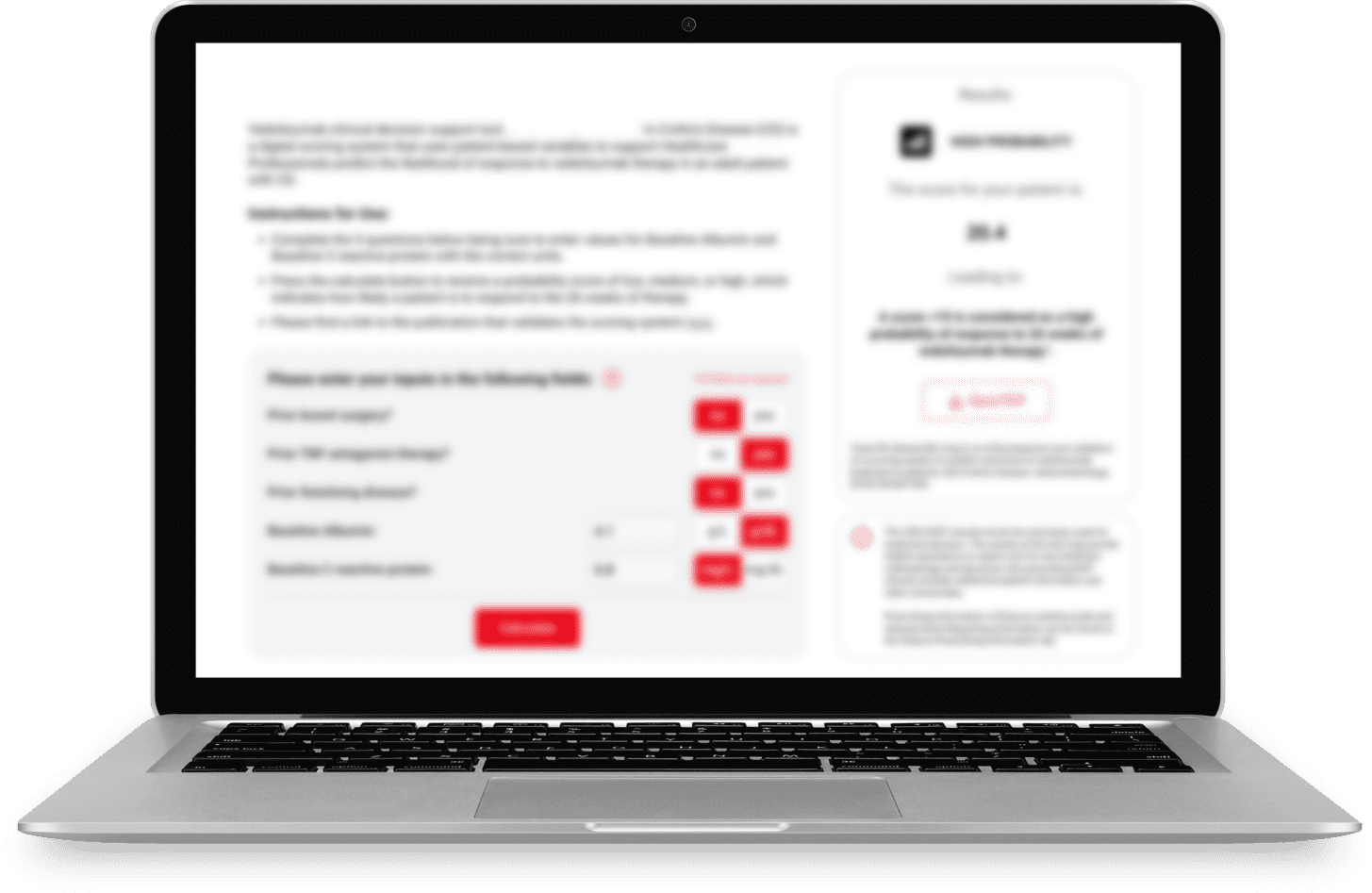
Hélène says that predictive models and CDSTs are one piece of a multistep process, which includes physician assessments and patient preferences, to choose the most appropriate therapies and optimize treatment effectiveness on an individualized basis in routine practice.1 “It can also help anticipate the probability regarding how the patient could respond to the treatment and, with their physician, make an informed decision,” she says.
A patient companion that can help a patient and their physician personalize treatment in hemophilia
Tim explains, “It’s incredibly rewarding to work on products like these. Hemophilia patients are often diagnosed at a young age, and their pharmacokinetic (PK) profile, which measures how medicine moves through their body, evolves over time, requiring periodic PK re-assessments to determine their treatment needs. This used to require up to 11 blood draws over the course of a few days4.”
Guiding self-injections to build confidence
Technological advances and pressures to health care systems globally have produced a significant shift toward home administration of medicines. But self-injection comes with challenges, such as a fear of needles, concerns about pain and a lack of confidence in performing the injection correctly.5
Recognizing this trend, Takeda is developing core technology and software to better support patients with their home injections. The system is designed as a complete self-dosing ecosystem that includes advanced injector technology, smart packaging and a user-friendly companion smartphone app.
Tim says this new technology could help guide patients through the process of administering their treatment. “For example, it is designed to provide real-time feedback that would let them know if they’re injecting correctly, and it can automatically record every injection. We continue to explore potential features to enhance the ease of patient administration.”
The concept can be adapted to help a potentially large population of patients track their injections and gain insights that could help manage their treatments.
Streamlining development to expand the use of SaMD

Jaclyn Loushine, director of Precision Medicine and Digital Health
When software crosses into the territory of being a medical device, it requires review from regulatory agencies such as the U.S. Food and Drug Administration (FDA) and European Union notified bodies, before being made available for commercial use. Achieving regulatory acceptance for SaMDs requires robust quality controls and testing, which sets a high standard for companies entering the space. Jaclyn Loushine, director of Precision Medicine and Digital Health at Takeda, says establishing a regulatory strategy for a SaMD is often complex.
“The regulatory frameworks in place for SaMDs are different across geographies, and the regulatory evidence needed to support bringing a SaMD to market is highly dependent on the risk associated with its intended use. Because of this, the path to market and timelines for developing SaMDs can vary significantly.”
Even so, Tim says taking a leap to begin the research and development process is necessary to truly impact patient health.
“Once we’ve established the regulatory pathway and the technology, it’s easier to expand SaMD into other therapeutic areas,” he explains. “The hope is to develop more SaMD solutions faster to complement therapies earlier in their life cycle. The good news is that other Takeda teams with therapies in late-stage development are doing important work in this space, too.”
Sriman concludes: “The realm of SaMD is emerging, and quality and regulatory functions will be crucial in navigating and structuring the registration process and geographic expansion into new markets.
“We’re excited to use SaMD solutions to help personalize experiences and we hope it can contribute toward making a world of difference to both patients and providers.”
References
- Dulai PS et al. (2018) Gastroenterology 155:687-695.e10.
- Dulai PA, et al. (2020) Ailment Pharmacol Ther.51:553‒564.
- Al-Bawardy B, Frontiers in Pharmacology, 12. doi:10.3389/fphar.2021.651415.
- Iorio A, et al. J Thromb Haemost. 2017;15(12):2461–2465. https://www.jthjournal.org/action/showPdf?pii=S1538-7836%2822%2904418-X
- Demographic, Physical, and Psychological Determinants of Patient Experience with Subcutaneous Self-Injection in Patients with Rheumatoid Arthritis: Structural Equation Modeling Approach: https://www.dovepress.com/demographic-physical-and-psychological-determinants-of-patient-experie-peer-reviewed-fulltext-article-PPA#ref-cit0018)
Share this story



