COVID-19 and the Role of Diagnostic Testing for Asymptomatic and Pre-Symptomatic Individuals
COVID-19 and the Role of Diagnostic Testing for Asymptomatic and Pre-Symptomatic Individuals
The COVID-19 pandemic is continuing to drive an unprecedented and increasing demand for diagnostic testing. The coming months are shaping up to be potentially challenging to the Health Care System as the desire to sustain reopened schools1 and businesses collides with the respiratory virus and influenza season alongside a potential resurgence in COVID-19 cases. While testing is a critical component involved in minimizing transmission, it’s not the only factor; other public health initiatives are critical including social distancing, masking, etc., which can, and have, had a dramatic impact on spread of disease. And yet, as we learn about asymptomatic and pre-symptomatic patients’ ability to infect2 and the potential for long-term deleterious effects of this disease3, it remains evident that there is a need for more frequent and accessible screening for asymptomatic individuals and testing for diagnostic purposes to prevent transmission and manage disease.
As a nation, the United States increased testing capacity for COVID-19 from near zero in early March to approximately 1 million tests today (Figure 1). As a Country and as an Industry we need to increase testing capacity and “widen the road” so to speak. Governments play a key role in all aspects of testing including facilitating fast approvals of good tests, and providing financial incentives to labs to expand quickly. We need to take a coordinated response to continue to increase capacity and access the resources necessary to get results back in less than 48 hours; while simultaneously expanding viral targets within COVID-19 tests to include seasonal respiratory viruses including influenza and Respiratory Syncytial Virus (RSV), diseases that have similar symptoms to COVID-19, to prepare for the upsurge. Laboratory capacity constraints may further be exacerbated as individuals who chose to defer testing for other diseases during the upsurge of the pandemic, will no longer be able to postpone testing. In cases of cancer, laboratories saw a testing decrease of 15-25% over 3 months4. Here we propose a series of recommendations to help our nation meet this unprecedented testing need through the integration of testing strategies and tactic into other policy considerations.
Figure 1. Number of COVID-19 tests per day in the US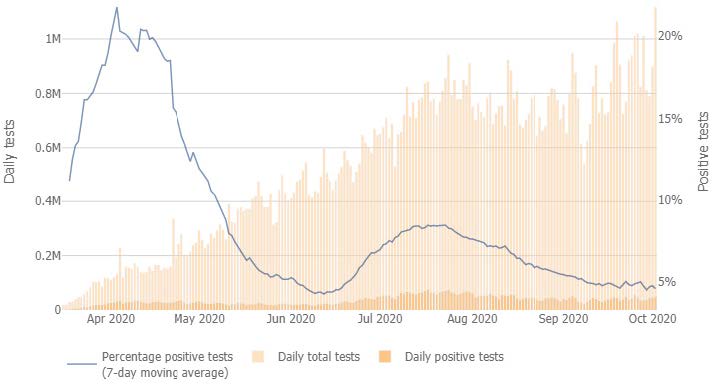
https://coronavirus.jhu.edu/testing/individual-states
SAMPLE POOLING
Laboratories can significantly increase testing capacity through the use of sample “pooling”. Patient sample pooling, the process by which multiple patient samples are combined before testing, is commonly viewed by diagnostic experts as the most viable and straightforward path to increasing testing capacity from our current state. However, this approach only makes sense from an economical and testing capacity perspective if 1) the positivity rate is below a certain threshold, and 2) the pooling size is not too large5 (i.e. 4 sample pooling leads to a 4-fold increase in limit of detection). Limit of detection, or LOD, is a measure of how many copies of virus must be present in a sample for a test to report a positive result. It has been estimated that every 10-fold increase in test LOD, leads to a 13% increase in false negative rate6. Provided the sensitivity of the assay is sufficient and the prevalence of positive patients is sufficiently low enough, pooling presents a viable mechanism for reducing the burden on the testing system7.
Sampling
There is also a tremendous burden on our front-line clinical workers, many of whom are stretched beyond reasonable limits. We will also need to improve how we prioritize the deployment of our clinicians and resources. We can reduce the demand on clinicians, while concurrently minimizing the risk of health care worker exposure through the utilization of alternate self-collection devices (e.g. saliva, nasal swab), which are consumer friendly, pain-free alternatives to replace previously used collections, which some refer to affectionately as “brain scratching”. We believe that the discomfort and fear associated with the initial sampling approaches for testing has negatively impacted the willingness of individuals to get tested.
This diversification of collection approaches will go a long way toward alleviating critical supply chain demands for testing resources, and “fit for purpose” deployment of these capabilities should be assessed.
ENSURING HIGH QUALITY TESTING THROUGH A LAB PROFICIENCY PROGRAM
Review of the Emergency Use Authorizations (EUA) filings with the FDA for protein antigen and PCR tests show that almost all systems report similar positive and negative agreement values of >95%. However, we know that false negatives and false positives are significant concerns in testing today, despite the reported high sensitivity and specificity. An alternative approach is needed to ensure confidence in the quality of COVID testing results.
Traditionally in the laboratory testing industry, testing proficiency programs administered by a central government agency like the Centers for Medicare and Medicaid Services (CMS) are used to ensure high quality test results. Proficiency programs consist of CMS sending blinded samples to clinical laboratories to demonstrate performance. We recommend that a similar proficiency approach be implemented for SARS-CoV-2 for both laboratories and providers of Point of Care devices - so that a minimum quality standard can be established, along with a true estimation of test sensitivity and specificity.
CENTRALIZED SAMPLE ROUTING, REPORTING, AND CONTACT TRACING
A centralized, national system for results reporting, sample routing and contract tracing is a must. Laboratories are currently being required to report at both state and local levels. With 50 states and 3,141 counties, many with differing requirements, the burden is too great and costly.
The nation is not currently fully maximizing existing laboratory capacity. Often times some laboratories have sample testing backlogs while others sit idle. A centralized system for routing samples to laboratories with excess capacity and shorter turnaround times could help alleviate “sample traffic jams”.
Prescriptions could be eliminated and instead the centralized system for results reporting and sample routing could also be used for patient follow-up and contact tracing. There is little to no difference in viral load and likely associated transmissibility in pre-symptomatic, asymptomatic or symptomatic patients, thus reducing viral spread can’t just be limited to just symptomatic patients diagnosed by a physician. Pre-symptomatic transmission is likely occurring in approximately 50% of the cases2. Eliminating prescriptions will greatly promote broader access and help control virus spread.
PROMOTING NEW TEST DEVELOPMENT AND INCREASED LABORATORY TESTING CAPACITY
The creation of a centralized sample repository, administered by the FDA or CDC, would accelerate new test development. This repository should include SARS-CoV-2, influenza, and RSV specimens in a variety of different collection media to support the creation of robust tests to diagnose these similar-symptom diseases, either as standalone tests or in combination.
Finally, we need to find unique ways to support additional investment in people, systems and reagents across the testing landscape; traditional “research funding” may not be the most effective mechanism. Existing laboratories and diagnostic companies must be encouraged to make capital investments and rapidly expand their capacity to respond to pandemics such as COVID-19. One suggestion would be to create governmentbacked upfront, low interest, forgivable loans tied to testing capacity increases achieved. For example, CLIA laboratories or diagnostic companies could be eligible for loans equal to 25% of their quarterly projected COVID volume increase. A laboratory that agrees to expand its capacity or test production by 100,000 tests per quarter would be eligible for a $2.5 million dollar loan (100,000 x $100 per test x 0.25 = $2,500,000) at 1% interest rate. All, or a portion, of the loan could be forgiven based upon the increase in testing volume that is achieved. Another incentive would be a government program which pre-purchases diagnostic tests, which could fund research and expansion in subsequent periods.
CONCLUSIONS
There is no single “magic bullet” to address the complexities of a pandemic like COVID-19. Scientific knowledge of the disease is unfolding daily. The uniqueness of our nation’s healthcare and political structures bear a diversity that is not dissimilar to country to country differences in other parts of the globe. We advocate the need for a more centralized, broader, holistic approach that extends from science to public policy.
In summary, our shared recommendations include:
- Implementation of a more diverse sampling approach which includes nasal swabs, saliva, etc. in addition to nasopharyngeal swabs, both with centralized collection as well as self-collected sample specimens and pooling, where appropriate.
- Development of a nationally administered proficiency and quality program to evaluate real-world test performance and set minimum performance requirements.
- Creation of a centralized system for results reporting, sample routing and contact tracing.
- Elimination of prescription requirements for COVID-19 testing in all jurisdictions.
- Creation of a central repository of sample specimens to aid test developers in developing combination SARS-CoV-2, influenza and RSV tests.
- Establishment of a program for low interest, forgivable government loans or “prepayment” for testing to support capacity expansion investments by qualified laboratories and test manufacturers. The US must act quickly if it wants to move from a reactionary position into one of leadership in response to this pandemic thus setting a precedent for effective management of this and future pandemics.
*The consortium was organized and is led by Bristol Myers Squibb. Consortium members include:
- Alnylam Pharmaceuticals, Inc.
- ArcherDx, Inc.
- Biocartis NV
- Color
- Diaceutics, PLC
- DxTerity Diagnostics, Inc.
- Genosity, Inc.
- GlaxoSmithKline, LLC
- Hematogenix Laboratory Services
- Infinity BiologiX
- Interpace Pharma Solutions, Inc.
- Janssen Pharmaceutica, NV
- LabCorp
- NeoGenomics Laboratories, Inc.
- Novartis Institute for BioMedical Research
- Ortho-Clinical Diagnostics, Inc.
- Syngene International Limited
- Takeda Pharmaceutical Company Limited (“Takeda”)
REFERENCES
- Map: Coronavirus and School Closures in 2019-2020. Accessed September 30, 2020.
https://www.edweek.org/ew/section/multimedia/map-coronavirus-and-school-closures.html - Walsh KA, Jordan K, Clyne B, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection.
J Infect. 2020;81(3):357-371. doi:10.1016/j.jinf.2020.06.067 - Couzin-Frankel, J. From ‘brain fog’ to heart damage, COVID-19’s lingering problems alarm scientists. July 31, 2020, 1:30 PM. Accessed September 30 2020. https://www.sciencemag.org/news/2020/07/brain-fog-heart-damage-covid-19-s-lingering-problems-alarm-scientists
- Cancer Testing and Diagnosis Suffers Sharp Downturn, finds Diaceutics. April 29,2020. Accessed October 5,2020.
https://www.diaceutics.com/cancer-testing-and-diagnosis-suffers-sharp-downturn-finds-diaceutics/ - Eberhardt JN, Breuckmann NP, Eberhardt CS. Multi-Stage Group Testing Improves Efficiency of Large-Scale COVID-19 Screening. J Clin Virol. 2020;128:104382. doi:10.1016/j.jcv.2020.104382
- Arnaout R, Lee RA, Lee GR, et al. SARS-CoV2 Testing: The Limit of Detection Matters. Preprint. bioRxiv. 2020;2020.06.02.131144. Published 2020 Jun 4. doi:10.1101/2020.06.02.131144
- Cleary B, Hay JA, Blumenstiel B, Gabriel S, Regev A, Mina MJ. Efficient prevalence estimation and infected sample identification with group testing for SARS-CoV-2. Preprint. medRxiv. 2020;2020.05.01.20086801. Published 2020 May 6. doi:10.1101/2020.05.01.
